
Recyclable catalysts
Controlling selectivity in alkene oxidation: anion driven epoxidation or dihydroxylation catalyzed by [Iron(III)(Pyridine-Containing Ligand)] complexes
G. Tseberlidis, L. Demonti, V. Pirovano, M. Scavini, S. Cappelli, S. Rizzato, R. Vicente, A. Caselli ChemCatChem, 2019, 11, 4907-4915[Link]
Abstract:
A highly reactive and selective catalytic system comprising Fe(III) and macrocyclic pyridine-containing ligands (Pc-L) for alkene oxidation by using hydrogen peroxide is reported herein. Four new stable iron(III)
complexes have been isolated and characterized. Importantly, depending on the anion of the iron(III) metal complex employed as catalyst, a completely reversed selectivity was observed. When X=OTf, a selective
dihydroxylation reaction took place. On the other hand, employing X=Cl resulted in the epoxide as the major product. The reaction proved to be quite general, tolerating aromatic and aliphatic alkenes as well
as internal or terminal double bonds and both epoxides and diol products were obtained in good yields with good to excellent selectivities (up to 93% isolated yield and d.r.=99:1). The catalytic system proved
its robustness by performing several catalytic cycles, without observing catalyst deactivation. The use of acetone as a solvent and hydrogen peroxide as terminal oxidant renders this catalytic system appealing.
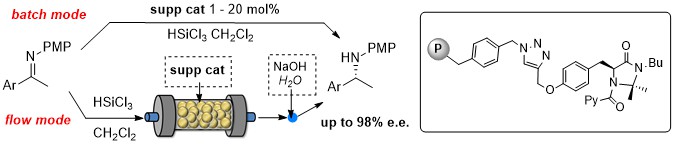
Solid supported chiral N-picolylimidazolidinones: recyclable catalysts for the enantioselective, metal- and H2-free reduction of imines in batch and in flow mode
R. Porta, M. Benaglia, R. Annunziata, A. Puglisi, G. Celentano Adv. Synth. Catal, 2017, 359, doi:10.1002/adsc.201700376 [Link]
Abstract:
A new class of solid supported chiral imidazolidinones organocatalysts for the catalytic reduction of imines with trichlorosilane
was developed. Polystyrene proved to be a more effective support than silica in terms of both chemical and stereochemical efficiency.
Even with a loading as low as 1 mol % the best performing supported catalyst showed a remarkable activity and stereocontrol ability,
promoting the reduction with stereoselectivities reaching 98% e.e. and in most cases ranging between 90-95% e.e. The general scope of
the methodology and the good recyclability of the immobilized catalyst were demonstrated. The polystyrene-anchored chiral catalyst was
also used to prepare packed bed reactors for the continuous flow synthesis of chiral amines, that were obtained in excellent yields and
enantioselectivities. By exploiting the chiral organocatalytic reactor, the in-flow stereoselective synthesis of a common, immediate
precursor of rivastigmine, of the calciomimetic (R)-NPS 568 and of Acrylamide (S)-A, currently under study for the treatment of
neuropathic pain, was successfully accomplished.
Comparison of Different Polymer- and Silica-Supported 9-Amino-9-deoxy-epi-quinines as Recyclable Organocatalysts.
R. Porta, F. Coccia, R. Annunziata and A. Puglisi, ChemCatChem, 2015, 7, 1490-1499. [Link]
Abstract:
9-Amino-9-deoxy-epi-quinine, properly modified by suitable linkers, was anchored on highly cross-linked polystyrene,
poly(ethylene glycol), and silica. The resulting species were characterized by NMR spectroscopy and tested as supported
organocatalysts in the reaction between isobutyric aldehyde and trans-ßnitrostyrene. Polystyrene- and poly(ethylene glycol)-supported
catalysts outperformed their nonsupported counterpart affording the desired product in high yield and ee (>90% ee).
Silica-supported catalysts proved to be less efficient in terms of both chemical yield and enantioselectivity. Polystyrene- and
poly(ethylene glycol)-supported 9-amino-9-deoxy-epi-quinine were then used in the same reaction with different substrates, leading
to the desired products in high yield and ee, as well as in three other reactions operating with different mechanism. An investigation
of the recyclability of the polystyrene- and poly(ethylene glycol)-supported systems showed that these could be recovered and recycled
with no loss of stereochemical activity but with a marked erosion of chemical efficiency occurring at the fifth reaction cycle. This was
ascribed to chemical degradation of the alkaloid occurring during the reaction.
Immobilization of γ-Glutamyl Transpeptidase from Equine Kidney for the Synthesis of kokumi Compounds
M. Bruni, M.S. Robescu, D. Ubiali, G. Marrubini, R. Vanna, C. Morasso, I. Benucci, G. Speranza, T.Bavaro
ChemCatChem, 2020, 12 210-128 [Link]
Abstract:
γ-Glutamyl transpeptidase from equine kidney (ekGGT, E.C. 2.3.2.2) is an intrinsic membrane enzyme which transfers
the γ-glutamyl moiety of glutathione to amino acids and peptides, thus producing γ-glutamyl derivatives.
An immobilization study of ekGGT was carried out with the aim to develop a robust biocatalyst for the synthesis
of γ-glutamyl amino acids which are known as kokumi compounds. Heterofunctional octylglyoxyl-agarose resulted in a
high immobilization yield and activity recovery (93% and 88%, respectively). Immobilized ekGGT retained more than 95%
activity under reaction conditions (Tris-HCl, pH 9, 0.05 M) after 6 days, whereas the residual activity after 6 reaction
cycles (18 days) was 85%. The synthesis of γ-glutamylmethionine catalyzed by octyl-glyoxylagarose-ekGGT afforded the product
in 42% yield (101 mg). The immobilized ekGGT was characterized by Raman spectroscopy. The immobilization protocol
developed for ekGGT could be of general applicability to membrane proteins.
Synthesis of Adenine Nucleosides by Transglycosylation using Two Sequential Nucleoside Phosphorylase-Based Bioreactors with On-Line Reaction Monitoring by using HPLC
G. Cattaneo, M. Rabuffetti, G. Speranza, T. Kupfer, B. Peters, G. Massolini, D. Ubiali, E. Calleri
ChemCatChem, 2017, 9 4614–4620 [Link]
Abstract:
Uridine phosphorylase from Clostridium perfringens (CpUP, EC 2.4.2.3) was immobilized covalently in an aminopropylsilica
monolithic column (25 mmV4.6 mm) upon functionalization with glutaraldehyde. Imino bonds that result from the reaction
between the enzyme and the support were reduced chemically to afford a 66% yield (13 mg) determined spectrophotometrically.
The CpUP immobilized enzyme reactor (IMER) was connected to a silica particle-based IMER that contained a purine nucleoside
phosphorylase from Aeromonas hydrophila (AhPNP, EC 2.4.2.1), which was developed previously and used successfully for the
fast synthesis of some purine ribonucleosides by a “one-enzyme” transglycosylation. CpUP-IMER and AhPNP-IMER were connected
to a HPLC system by a six-way switching valve. In this set-up, the synthesis of 2’-deoxyadenosine (dAdo, 8), adenosine
(Ado, 9), and arabinosyladenine (araA, 10) by a “two-enzyme” transglycosylation is coupled directly to on-line reaction
monitoring. Under the optimized transglycosylation conditions (2:1 ratio sugar donor/base acceptor; 10 mm
phosphate buffer; pH 7.25; temperature 37 8C, flow rate 0.1 mLmin@1), defined by experimental design, the conversion of
dAdo and Ado was approximately 90 %, and araA was synthesized in 20% yield.